Severely
Damaged Mitochondria and Defective Krebs cycle and ATP production
Source: ME Primer for Healthcare Professionals: based on Myalgic encephalomyelitis: International Consensus Criteria, 2012
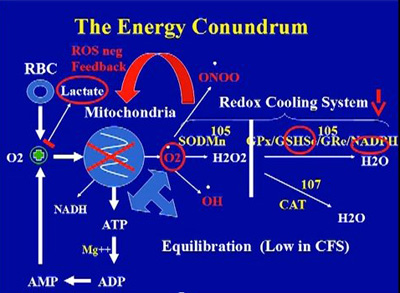
Source: Dr. Paul Cheney, The Cheney Clinic, USA.
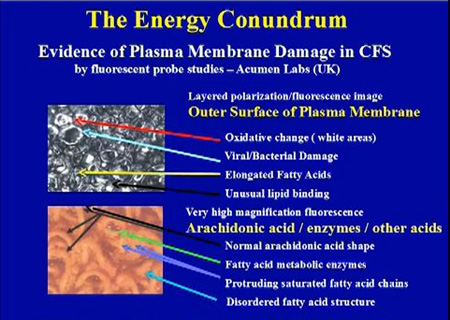
Source: Dr. Paul Cheney, The Cheney Clinic, USA.
Cardiomyopathy arising from mitochondria dysfunction, oxidative stress and infections
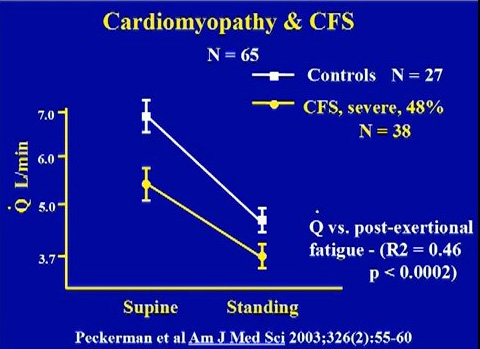
Source: Peckerman et al. 2003
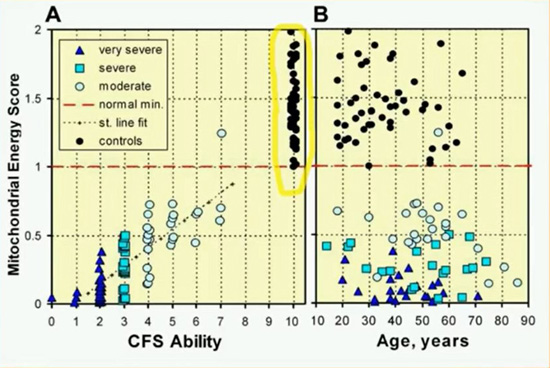
Source: Dr. Sarah Myhill. Myhill Medical Clinic, Wales.
- The
cristae (the infoldings of the inner membrane of mitochondria) have
gone, leaving honeycombed patterns. This suggests a toxic or stress
phenomeon in the mitochondria.
Mitochondrial abnormalities in PVFS. Acta Neuropathalogica, 1991,
83, 61-65.
Professor Peter Behan, The Institute of Neurological Sciences,
University of Glasgow, Scotland)
- "Unusual
pattern of mitochondrial DNA deletions in skeletal muscle of an
adult human with chronic fatigue syndrome,"
Hum Mol Genet, 1995 April, 4(4) ), McCully (US), Suhadolnik (US) and
Brad J. Chazotte
- Mitochondria DNA and Gene tests
These are listed in the following paper
Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome.
J Transl Med. 2016 Jan 20;14(1):19. doi: 10.1186/s12967-016-0771-6. Billing-Ross P1, Germain A2, Ye K3, Keinan A4, Gu Z5, Hanson MR6.
-
Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Norman E Booth, Sarah Myhill, John McLaren-Howard. Oxford University. Int J Clin Exp Med 2012;5(3):208-220 www.ijcem.com /ISSN:1940-5901/IJCEM1204005. (2012)
-
Chronic fatigue syndrome and mitochondrial dysfunction. Sarah Myhill, Norman E. Booth, John McLaren-Howard. Int J Clin Exp Med (2009) 2, 1-16 www.ijcem.com/IJCEM812001
- Targeting mitochondrial dysfunction in the treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) – a clinical audit. Sarah Myhill et al. Int J Clin Exp Med 2013;6(1):1-15.
- Cell Metabolics
- Metabolic Features of Chronic Fatigue Syndrome. Naviaux et al. 2016
- Research by the Open Medicine Foundation in California (Dr. Ron Davis and others)
- The research papers and findings are listed on http://www.openmedicinefoundation.org
- The current findings (2016) indicate severe disruption of the citric acid cycle, inside mitochondria, the source of energy for the body. The mitochondria are functioning abnormally and not producing enough ATP. They have partially shut down and this explains the chronic exhaustion and lack of energy in patients.
- Also glucose is being converted into fatty acids not energy.
- There is deficiency of biotin.
- The GTP cyclohydrolase pathway is very low in ME/CFS, and downstream from that, as a consequence BH4 also seems to be low. This has multiple adverse effects on the human body.
- Lane RJ, Barrett MC, Taylor DJ, Kemp GJ, Lodi R: Heterogeneity in chronic fatigue syndrome: evidence from magnetic resonance spectroscopy of muscle. Neuromuscul Disord 1998, 8:204-209.
- Behan WMH, Holt J, Kay DH, Moonic P (1999), "In vitro Study of Muscle Aerobic Metabolism in Chronic Fatigue Syndrome", Journal of Chronic Fatigue Syndrome
- The assessment of the energy metabolism in patients with chronic fatigue syndrome by serum fluorescence emission.
Mikirova N, Casciari J, Hunninghake R.Altern Ther Health Med. 2012 Jan-Feb;18(1):36-40.
- Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial
dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic
fatigue syndromes and fibromyalgia patients: peripheral and central me
chanisms as
therapeutic targets?
Expert Opin Ther Targets
(
2013
)
17
:
1081
-
1089. doi:
10.1517/14728222.2013.818657.
- Pietrangelo T, Mancinelli R, Toniolo L, Toniolo L, Vecchiet J, Fan๒ G, et al.
Transcription profile analysis of vastus lateralis muscle from
patients with chronic
fatigue syndrome.
Int J Immunopathol Pharmacol
(
2009
)
22
:
795
-
807.
- Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue
syndrome.
Acta Neuropathol
(
1991
)
83
:
61
-
65. doi: 10.1007/BF00294431.
- CAV
virus infection of the mitochondria
"CAV-infected cells could be characterized by electron-dense
circular virions, some with electron-luscent cores and others with
electron-dense cores, associated with the rough endoplasmic
reticulum and inside large abnormally distended mitochondria in the
cells."
World patent entitled "Method and Compositions for Diagnosing
and Treating Chronic Fatigue Immunodysfunction Syndrome"
#WO9205760 issued to Elaine DeFreitas and Brendan Hilliard,
inventors assigned to Wistar Institute, USA. This patent was applied
for in August 1991. It concerns the discovery of a new virus the CAV
virus which may lie at the root of CFS / ME.
-
"A
growing body of evidence indicates that mitochondrial dysfunction
may play an important role in the pathogenesis of many
neurodegenerative diseases," he quoted (Brain Path, 2000 Jul,
10(3), Manfredi, Beal),...acquired mitochondrial defects could be
the cause of neuronal degeneration as a consequence of energy
defects and oxidative damage...understanding the role of
mitochondria in the pathogenesis of neurodegenerative diseases could
be important for the development of therapeutic strategies in these
disorders." "Electrophysiological evidence of central
nervous system involvement is present in a high number of patients
with mitochondrial disorders (Electroencephalogr Clin Neurophysiol,
1997 Jun, 105(3) DiLazzaro et al).
1/3
of the Mitochondria's DNA is missing in ME / CFIDS ! The
undulating protein, called criti, effectively can hide the
retrovirus. The would effect your entire body, patients are advised
to plot their CD cells (CD 4, CD8, CD19).
Professor Alan Cocchetto, National CFIDS Foundation, USA. http://www.ncf-net.org
Beckman et al. reported
that peroxynitrite reacts with and inactivates several important
mitochondrial enzymes leading to metabolic energy dysfunction
(Beckman et al. 1993; Radi et al. 1994), characteristics of both CFS
and MCS. This ties in with Dr. Martin Pall's Peroxynitrite theory.
From Neurology to Mitochondia. Richardson J, Costa DC. J CFS 1998, 4(3)
- Brown GC, Borutaite V (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658(1–2):44–9
- They used global transcriptome analysis to identify genes that were differently expressed in the vastus lateralis, and their results are significant. They found that the expression of genes that play key roles in mitochondrial function and oxidative balance (including superoxide dismutase) were altered in ME/CFS patients. Other genes that were altered in these patients include the genes involved in energy production, muscular trophism and fibre phenotype determination.Importantly, the expression of a gene encoding a component of the nicotinic cholinergic receptor binding site was reduced, suggesting impaired neuromuscular transmission. The authors argue that these major biological processes could be involved in and/or responsible for the muscle symptoms of ME/CFS (Int J Immunopathol Pharmacol 2009:22(3):795-807).
-
“Ten patients with post-viral fatigue syndrome and abnormal serological, viral, immunological and histological studies were examined by single fibre electromyographic technique….The findings confirm the organic nature of the disease. A muscle membrane disorder…is the likely mechanism for the fatigue and the single-fibre EMG abnormalities. This muscle membrane defect may be due to the effects of a persistent viral infection…There seems to be evidence of a persistent viral infection and/or a viral-induced disorder of the immune system…The infected cells may not be killed but become unable to carry out differentiated or specialised function” (Goran A Jamal, Stig Hansen. Euro Neurol 1989:29:273-276).
- “Myalgic encephalomyelitis is a common disability but frequently misinterpreted…This illness is distinguished from a variety of other post-viral states by a unique clinical and epidemiological pattern characteristic of enteroviral infection…33% had titres indicative and 17% suggestive of recent CBV infection…Subsequently…31% had evidence of recent active enteroviral infection…There has been a failure to recognise the unique epidemiological pattern of ME…Coxsackie viruses are characteristically myotropic and enteroviral genomic sequences have been detected in muscle biopsies from patients with ME. Exercise related abnormalities of function have been demonstrated by nuclear magnetic resonance and single-fibre electromyography including a failure to coordinate oxidative metabolism with anaerobic glycolysis causing abnormal early intracellular acidosis, consistent with the early fatiguability and the slow recovery from exercise in ME. Coxsackie viruses can initiate non-cytolytic persistent infection in human cells. Animal models demonstrate similar enteroviral persistence in neurological disease… and the deleterious effect of forced exercise on persistently infected muscles. These studies elucidate the exercise-related morbidity and the chronic relapsing nature of ME” (EG Dowsett, AM Ramsay et al. Postgraduate Medical Journal 1990:66:526-530).
- “Persistent enteroviral infection of muscle may occur in some patients with postviral fatigue syndrome and may have an aetiological role….The features of this disorder suggest that the fatigue is caused by involvement of both muscle and the central nervous system…We used the polymerase chain reaction to search for the presence of enteroviral RNA sequences in a well-characterised group of patients with the postviral fatigue syndrome…53% were positive for enteroviral RNA sequences in muscle…Statistical analysis shows that these results are highly significant…On the basis of this study…there is persistent enteroviral infection in the muscle of some patients with the postviral fatigue syndrome and this interferes with cell metabolism and is causally related to the fatigue” (JW Gow et al. BMJ 1991:302:696-696).
- “The findings described here provide the first evidence that postviral fatigue syndrome may be due to a mitochondrial disorder precipitated by a virus infection…Evidence of mitochondrial abnormalities was present in 80% of the cases with the commonest change (seen in 70%) being branching and fusion of cristae, producing ‘compartmentalisation’. Mitochondrial pleomorphism, size variation and occasional focal vacuolation were detectable in 64%…Vacuolation of mitochondria was frequent…In some cases there was swelling of the whole mitochondrion with rupture of the outer membranes…prominent secondary lysosomes were common in some of the worst affected cases…The pleomorphism of the mitochondria in the patients’ muscle biopsies was in clear contrast to the findings in normal control biopsies…Diffuse or focal atrophy of type II fibres has been reported, and this does indicate muscle damage and not just muscle disuse” (WMH Behan et al. Acta Neuropathologica 1991:83:61-65).
- “The findings described here provide the first evidence that postviral fatigue syndrome may be due to a mitochondrial disorder precipitated by a virus infection…Evidence of mitochondrial abnormalities was present in 80% of the cases with the commonest change (seen in 70%) being branching and fusion of cristae, producing ‘compartmentalisation’. Mitochondrial pleomorphism, size variation and occasional focal vacuolation were detectable in 64%…Vacuolation of mitochondria was frequent…In some cases there was swelling of the whole mitochondrion with rupture of the outer membranes…prominent secondary lysosomes were common in some of the worst affected cases…The pleomorphism of the mitochondria in the patients’ muscle biopsies was in clear contrast to the findings in normal control biopsies…Diffuse or focal atrophy of type II fibres has been reported, and this does indicate muscle damage and not just muscle disuse” (WMH Behan et al. Acta Neuropathologica 1991:83:61-65).
- We performed histochemical and quantitative analysis of enzymatic activities and studies of mitochondrial DNA deletions. All specimens showed hypotrophy, fibres fragmentation, red ragged fibres, and fatty and fibrous degeneration. Electron microscopy confirmed these alterations, showing degenerative changes, and allowed us to detect poly/pleomorphism and cristae thickening of the mitochondria. The histochemical and quantitative determination of the enzymatic activity showed important reduction, in particular of the cytochrome-oxidase and citrate-synthetase. The ‘common deletion’ of 4977 bp of the mitochondrial DNA was increased as high as 3,000 times the normal values in three patients. Our results agree with those of Behan et al 1991 and Gow et al 1994. The alterations are compatible with a myopathy of probable mitochondrial origin (which) could explain the drop in functional capability of the muscle ” (JCFS 1996:2:(2/3):76-77)
- Scientific studies into the harmful effects of Roundup and GMO foods
- Vermeulen RCW, Kurt RM, Visser FC, Sluiter W, Scholte HR: Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med 2010, 8:93.
- Weinstein AA, Drinkard BM, Diao G, Furst G, Dale JK, Straus SE, et al. Exploratory
analysis of the relationships between aerobic capacity and self
-
reported fatigue in
patients with rheumatoid arthritis, polymyositis, and chronic
fatigue syndrome.
PM R
(
2009
)
1
:
620
-
628. doi: 10.1016/j.pmrj.2009.04.007
- Arnold DL, Bore PJ, Radda GK, Styles P, Taylor DJ: Excessive intracellular acidosis of skeletal muscle on exercise in a patient with a post-viral exhaustion/fatigue syndrome. Lancet 1984, 1:1367-1369
- Cea G, Bendahan D, Manners D, Hilton-Jones D, Lodi R, Styles P, Taylor DJ (2002), "Reduced oxidative phosphorylation and proton efflux suggest reduced capillary blood supply in skeletal muscle of patients with dermatomyositis and polymyositis: a quantitative 31P-magnetic resonance spectroscopy and MRI study", Brain Jul;125(Pt 7):1635-45,
- Dr.
Charles Shepherd cites several Research studies which show severe
and extensive damage to the mitochondria in ME / Chronic Fatigue
Syndrome patients. ' Living with ME - chronic/post-viral fatigue
sydrome ' by Dr. Charles Shepherd, Vermillion, ISBN 0-09-181679-3
- "Skeletal
oxidative damage was observed in CFS patients. This was thought to
be a consequence of imbalance between an abnormal production of
reactive oxygen species (due to oxidative metabolism related to
mitochondrial activity) and a disabled scavenger enzyme
system."
D. Racciatti (Chieti, Italy) research paper submitted to the AACFS
5th International Research, Clinical and Patient Conference, 2001
-
Lane RJM, Barrett MC, Woodrow D, Moss J, Fletcher R, Archard LC: Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 1998, 64:362-367
- Brad Chazotte, Department of Pharmaceutical Sciences, Campbell University, Buies Creek, North Carolina 27506. Mitochondria in Pathogenesis edited by Lemasters and Nieminen. Kluwer Academic/Plenum Publishers, New York, 2001.
- Behan WM, More IA, Downie I, Gow JW. Mitochondrial studies in the chronic fatigue syndrome. EOS Riv Immunol Immunofarmacol. 1995;15:36–9.
- Maes M (2009) Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry 22(1):75–83
- Wong R, Lopaschuk G, Zhu G, Walker D, Catellier D, Burton D, Teo K, Collins-Nakai R, Montague T: Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest 1992, 102:1716-1722
- Pietrangelo T, Mancinelli R, Toniolo L, Toniolo L, Vecchiet J, Fan๒ G, et al.
Transcription profile analysis of vastus lateralis muscle from
patients with chronic
fatigue syndrome.
Int J Immunopathol Pharmacol
(
2009
)
22
:
795
-
807.
- Mathew SJ, Mao X, Keegan KA, Levine SM, Smith EL, Heier LA, Otcheretko V, Coplan JD, Shungu DC: Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR Biomed 2009, 22:251-258
- Allen DG, Lamb GD, Westerbland H: Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 2008, 88:287-332.
- Vernon S, Whistler T, Cameron B, Hickie I, Reeves W, Lloyd A (2006), "Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr Virus", BMC Infect Dis. Jan 31;6 (1):15,
- Plioplys AV, Plioplys S: Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology 1995, 32:175-181
- Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 2014;29(1):19–36
- Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Maes et al. Curr Neuropharmacol. 2014 Mar;12(2):168-85.
- Rose S, Frye RE, Slattery J, Wynne R, Tippett M, Pavliv O, Melnyk S, James SJ (2014) Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a wellmatched case control cohort. PLoS One 9(1):e85436. doi:10.1371/ journal.pone.0085436. eCollection 2014
- Nagy G, Koncz A, Fernandez D, Perl A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic Biol Med. 2007;42:1625–31
- “Gene microarray data have led to better understanding of pathogenesis. Research has evaluated genetic signatures (and) described biologic subgroups. Genomic studies demonstrate abnormalities of mitochondrial function” (Curr Rheumatol Rep 2007:9(6):482-487)
- Presentation of Susan Levine from Columbia University at 7th AACFS International Conference showing several mitochondria abnormalities
- Impaired mitochondrial structure and function in ME/CFS patients (JNNP: 2003:74:1382-1386)
- Nestadt P et al reported neurobiological differences in (ME)CFS. These results show that a significant proportion of patients diagnosed with (ME)CFS have elevated ventricular lactate levels, suggesting anaerobic energy conversion in the brain and/or mitochondrial dysfunction. Elevated blood lactate levels after mild exercise are considered to be a sign of mitochondrial damage (IACFS International Research Conference, Florida, 2007).
- "Decreased levels inside the cell of a key enzyme called succinate dehydrogenase, which plays an important role in energy production inside the mitochondria (the power house of the cell)”. (Cambridge University Report, ME Association Newsletter, Autumn 1989, page 16.)
- “The pleomorphism of the mitochondria in the patients’ muscle biopsies was in clear contrast to the findings in the normal control biopsies. Diffuse or focal atrophy of type II fibres has been reported, and this does indicate muscle damage and not just muscle disuse”. (Acta Neuropathol 1991:83:61-65).
-
There
was evidence from British research that enteroviral RNA occurs in
the muscle tissue of CFS patients and this may lead to mitochondrial
injury
Gow J W et al. BMJ 1991; 302: 692
-
Gow
JW: Behan WM. Br Med Bull 1991; 47: 872
- Morris, G. & Maes, M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metabolic brain disease (2012).doi:10.1007/s11011-012-9324-8
- Pall, M. Explaining Unexplained Illnesses: Disease Paradigm for Chronic Fatigue Syndrome, Multiple Chemical Sensitivity, Fibromyalgia, Post-Traumatic Stress Disorder, Gulf War Syndrome and Others. (New York: Haworth Medical Press: 2007).
- Chazotte, B. Mitochondrial Dysfunction in Chronic Fatigue Syndrome. 393410 (Springer US: Boston, 2002).doi:10.1007/b114376
- Chaudhuri, A. & Behan, P. O. In vivo magnetic resonance spectroscopy in chronic fatigue syndrome. Prostaglandins, leukotrienes, and essential fatty acids 71, 1813 (2004).
- Wong, R. et al. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest 102, 171622 (1992).
- Lane, R. J., Barrett, M. C., Taylor, D. J., Kemp, G. J. & Lodi, R. Heterogeneity in chronic fatigue syndrome: evidence from magnetic resonance spectroscopy of muscle. Neuromuscular disorders?: NMD 8, 2049 (1998).
- McCully, K. K., Natelson, B. H., Iotti, S., Sisto, S. & Leigh, J. S. Reduced oxidative muscle metabolism in chronic fatigue syndrome. Muscle & nerve 19, 6215 (1996).
- Lane, R. J. et al. Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. Journal of neurology, neurosurgery, and psychiatry 64, 3627 (1998).
- Vecchiet, L. et al. Sensory characterization of somatic parietal tissues in humans with chronic fatigue syndrome. Neuroscience letters 208, 11720 (1996).
- Plioplys, A. V & Plioplys, S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology 32, 17581 (1995).
- Jammes, Y., Steinberg, J. G., Mambrini, O., Br้geon, F. & Delliaux, S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. Journal of internal medicine 257, 299310 (2005).
- Jammes, Y., Steinberg, J. G., Delliaux, S. & Br้geon, F. Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. Journal of internal medicine 266, 196206 (2009).
- Hollingsworth, K. G., Jones, D. E. J., Taylor, R., Blamire, A. M. & Newton, J. L. Impaired cardiovascular response to standing in chronic fatigue syndrome. European journal of clinical investigation 40, 60815 (2010).
- Jones, D. E. J. et al. Loss of capacity to recover from acidosis on repeat exercise in chronic fatigue syndrome: a case-control study. European journal of clinical investigation 42, 18694 (2012).
- Kaushik, N. et al. Gene expression in peripheral blood mononuclear cells from patients with chronic fatigue syndrome. Journal of clinical pathology 58, 82632 (2005).
- Jones DE, Hollingsworth KG, Jakovljevic DG, Fattakhova G, Pairman J, et al. Loss of capacity to recover from acidosis on repeat exercise in chronic fatigue syndrome: a case-control study. Eur J Clin Invest 2012;42(2):186–94
- Jones DE, Hollingsworth KG, Taylor R, Blamire AM, Newton JL. Abnormalities in pH handling by peripheral muscle and potential regulation by the autonomic nervous system in chronic fatigue syndrome. J Intern Med. 2010;267:394–401.
- Vermeulen, R. C. W., Kurk, R. M., Visser, F. C., Sluiter, W. & Scholte, H. R. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. Journal of translational medicine 8, 93 (2010).
- Vermeulen RC, Eck IW V v (2014) Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J Transl Med 12:20. doi:10.1186/1479-5876-12-20
- Castro-Marrero, J. et al. Could mitochondrial dysfunction be a differentiating marker between Chronic Fatigue Syndrome and Fibromyalgia? Antioxidants & redox signaling (2013).doi:10.1089/ars.2013.5346
- Mathew, S. J. et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR in biomedicine 22, 2518 (2009).
- Increased Ventricular Lactate
- Murrough, J. W. et al. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR in biomedicine 23, 64350 (2010).
- Shungu, D. C. et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR in biomedicine 25, 107387 (2012).
- Mathew SJ, Mao X, Keegan KA, Levine SM, Smith EL, Heier LA, et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR Biomed. 2009;22:251–8.
- Kuratsune, H. et al. Acylcarnitine deficiency in chronic fatigue syndrome. Clinical infectious diseases?: an official publication of the Infectious Diseases Society of America 18 Suppl 1, S627 (1994).
- Filler K, Lyon D, Bennett J, McCain N, Elswisk R, Lukkahatai N, et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23.
- Li, Y.-J. et al. Clinical characteristics of patients with chronic fatigue syndrome: analysis of 82 cases. Zhonghua yi xue za zhi 85, 7014 (2005).
- Okada, T., Tanaka, M., Kuratsune, H., Watanabe, Y. & Sadato, N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC neurology 4, 14 (2004).
- Soetekouw, P. M. et al. Normal carnitine levels in patients with chronic fatigue syndrome. The Netherlands journal of medicine 57, 204 (2000).
- Vermeulen, R. C. W. & Scholte, H. R. Exploratory open label, randomized study of acetyl- and propionylcarnitine in chronic fatigue syndrome. Psychosomatic medicine 66, 27682 (2004).
- Kurup, R. K. & Kurup, P. A. Hypothalamic digoxin, cerebral chemical dominance and myalgic encephalomyelitis. The International journal of neuroscience 113, 683701 (2003).
- Maes, M. et al. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro endocrinology letters 30, 4706 (2009).
- Lane RJ, Soteriou BA, Zhang H, Archard LC. Enterovirus related metabolic myopathy: a postviral fatigue syndrome. J Neurol Neurosurg Psychiatry. 2003;74:1382–6.
- Manuel y Keenoy, B. et al. Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. Journal of the American College of Nutrition 19, 37482 (2000).
- Nicolson, G. L. & Ellithorpe, R. Lipid Replacement and Antioxidant Nutritional Therapy for Restoring Mitochondrial Function and Reducing Fatigue in Chronic Fatigue Syndrome and other Fatiguing Illnesses. Journal of Chronic Fatigue Syndrome (2006).
- White, A. T., Light, A. R., Hughen, R. W., Vanhaitsma, T. A. & Light, K. C. Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosomatic medicine 74, 4654 (2012).
- Possible Impairment of PGC-1α causes rapid lactic acid build up in ME / CFS
- Summermatter S, Santos G, Pérez-Schindler J, Handschin C (2013) Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci U S A 110(21):8738– 43. doi:10.1073/pnas.1212976110
- .Bonen A (2009) PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34(3):307–14. doi:10.1139/H09-008 261.
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, BernalMizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC- 1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3(4):e101
- .Handschin C, Spiegelman BM (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454(7203):463–9. doi:10.1038/nature07206
- Austin S, St-Pierre J (2012) PGC1α and mitochondrial metabolism—emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125(Pt 21):4963–71. doi:10.1242/jcs. 113662 268.
- Light, A. R. et al. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. Journal of internal medicine 271, 6481 (2012).
- Light, A. R., White, A. T., Hughen, R. W. & Light, K. C. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. The journal of pain?: official journal of the American Pain Society 10, 1099112 (2009).
- Reduced levels of acyl-carnitine. Carnitine is used in mitochondria
oxydation.
Carnitine is essential for mitochondria health and function.
- Kuratsune H, Yamaguti K, Lindh G, Evengard B, Takahashi M, Machii T, Matsumura K, Takaishi J, Kawata S, Langstrom B, Kanakura Y, Kitani T, Watanabe Y (1998), "Low levels of serum acylcarnitine in chronic fatigue syndrome and chronic hepatitis type C, but not seen in other diseases", Int J Mol Med. Jul;2(1):51-6
- Research papers of Professor Peter Behan, The Institute of Neurological Sciences,
University of Glasgow, Scotland)
- Kuratsune H, Yamaguti K, Takahashi M, e.a. (1994), "Acylcarnitine deficiency in chronic fatigue syndrome", Clin Infect Dis, Jan;18 Suppl 1:S62-7
- Inazu M, Matsumiya T (June 2008). "[Physiological functions of carnitine and carnitine transporters in the central nervous system]". Nihon Shinkei Seishin Yakurigaku Zasshi (in Japanese) 28 (3): 113–20. PMID 18646596
- Malaguarnera M, Gargante MP, Cristaldi E et al. (2008). "Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue". Arch Gerontol Geriatr 46 (2): 181–90. doi:10.1016/j.archger.2007.03.012
- De Simone C, Famularo G, Tzantzoglou S, et al. Carnitine depletion in peripheral blood mononuclear cells from patients with AIDS: effect of oral L-carnitine. AIDS 1994;8:655-660
- Jones, M. G., Goodwin, C. S., Amjad, S. & Chalmers, R. A. Plasma and urinary carnitine and acylcarnitines in chronic fatigue syndrome. Clinica chimica acta; international journal of clinical chemistry 360, 173–7 (2005).
- Plioplys AV, Plioplys S. Serum levels of carnitine in chronic fatigue syndrome: clinical correlates. Neuropsychobiology 1995;32:132-138
- Plioplys AV, Plioplys S. Amantadine and L-carnitine treatment of chronic fatigue syndrome. Neuropsychobiology. 1997;35:16-23.
Famularo G, De Simone C. A new era for carnitine? Immunol Today 1995;16:211-2133
- Reuter, S. E. & Evans, A. M. Long-chain acylcarnitine deficiency in patients with chronic fatigue syndrome. Potential involvement of altered carnitine palmitoyltransferase-I activity. Journal of internal medicine 270, 76–84 (2011).
- Coenzyme Q10 Deficiency
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E: Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett 2009, 30:470-476.
- Judy W. Southeastern Institute of Biomedical Research, Bradenton, Florida. Presentation to the 37th Annual Meeting, American College of Nutrition, October 13, 1996.
- Vitamin E Deficiency
- Miwa K, Fujita M: Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels 2010, 25:319-323.
- Glutathione Deficiency
- Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, Medow MS, Natelson BH, Stewart JM, Mathew SJ: Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 2012, 25:1073-1087.
- Magnesium Deficiency
- Manuel y Keenoy B, Moorkens G, Vertommen J, Noe M, Neve J, De Leeuw I: Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. J Am Coll Nutr 2000, 19:374-382.
- Howard JM, Davies S, Hunnisett A. Magnesium and chronic fatigue syndrome. Letter. Lancet 1992;340:426.27.
- Manuel y Keenoy B, Moorkens G, Vertommen J, Noe M, Neve J, De Leeuw I: Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. J Am Coll Nutr 2000, 19:374-382.
- Chambers D, Bagnall AM, Hempel S, Forbes C (October 2006). "Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review". J R Soc Med 99 (10): 506–20. doi:10.1258/jrsm.99.10.506. PMC 1592057
- Cox IM, Campbell MJ, Dowson D (1991). "Red blood cell magnesium and chronic fatigue syndrome". Lancet 337 (8744): 757–60. doi:10.1016/0140-6736(91)91371-Z
- Grant JE, Veldee MS, Buchwald D. Analysis of dietary intake and selected nutrient concentrations in patients with chronic fatigue syndrome. J Am Diet Assoc 1996;96:383-386
- Cox IM, Campbell MJ, Dowson D. Red blood cell magnesium and chronic fatigue syndrome. Lancet 1991;337:757-760
- Howard JM, Davies S, Hunnisett A. Magnesium and chronic fatigue syndrome. Letter.Lancet 1992;340:426.
- Jessop, Carol – reported in the Fibromyalgia Network Newsletter compendium #2, October 1990-January 1992. 53.
- Moorkens G, Manuel Y, Keenoy B, et al. Magnesium deficit in a sample of the Belgian population presenting with chronic fatigue. Magnes Res 1997;10:329-337.
- Omega-3 oils and Zinc Deficiencies
- Maes M, Mihaylova I, Leunis JC: In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol Lett 2005, 26:745-751.
- Chambers D, Bagnall AM, Hempel S, Forbes C (October 2006). "Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review". J R Soc Med 99 (10): 506–20. doi:10.1258/jrsm.99.10.506. PMC 1592057
- Behan PO, Behan WM, Horrobin D (1990). "Effect of high doses of essential fatty acids on the postviral fatigue syndrome". Acta Neurol. Scand. 82 (3): 209–16. doi:10.1111/j.1600-0404.1990.tb04490.x
- Howard JM, Davies S, Hunnisett A. Magnesium and chronic fatigue syndrome. Letter. Lancet 1992;340:426
- Gray JB, Martinovic AM. Eicosanoids and essential fatty acid modulation in chronic disease and the chronic fatigue syndrome. Med Hypotheses 1994;43:31-42
- Kury PG, Ramwell PW, McConnell HM. The effect of prostaglandin E1 and E2 on the human erythrocyte as monitored by spin labels. Biochem Biophys Res Commun 1974;56:478-483
- Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS.Maes M, Mihaylova I, De Ruyter M. J Affect Disord. 2006 Feb;90(2-3):141-7. Epub 2005 Dec 9.
- Jessop, Carol – reported in the Fibromyalgia Network Newsletter compendium #2, October 1990-January 1992. 53.
- Research papers on Zinc in ME and CFS
- D-Ribose Deficiency
- Teitelbaum, J. E., Johnson, C. & St Cyr, J. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. Journal of alternative and complementary medicine (New York, N.Y.) 12, 857–62 (2006).
- Teitelbaum, J., Jandrain, J. & McGrew, R. Treatment of Chronic Fatigue Syndrome and Fibromyalgia with D-Ribose– An Open-label, Multicenter Study. The open pain journal 32–37 (2012).
- NADH Deficiency
- Forsyth LM, Preuss HG, MacDowell AL, Chiazze L, Birkmayer GD, Bellanti JA (February 1999). "Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome". Ann. Allergy Asthma Immunol. 82 (2): 185–91.
- Santaella, M. L., Font, I. & Disdier, O. M. Comparison of oral nicotinamide adenine dinucleotide (NADH) versus conventional therapy for chronic fatigue syndrome. Puerto Rico health sciences journal 23, 89–93 (2004).
- Alegre, J. et al. Nicotinamide adenine dinucleotide (NADH) in patients with chronic fatigue syndrome. Revista clínica española 210, 284–8 (2010).
ME outbreaks suggesting Mitochondria and Muscle abnormalities and role of viruses and other pathogens
Viruses have been isolated from the muscles of ME patients during epidemics. Using PCR methods, 50% or more of patients had evidence of such infection.
- Hyde BM.
Myalgic encephalomyelitis (chronic fatigue syndrome): an historic
perspective.
Can Dis Wkly Rep. 1991 Jan;17 Suppl 1E:5-8. PMID: 1669354
- Brodrick J.
Myalgic encephalomyelitis: yuppie 'flu--a real illness. Interview by Charlotte
Alderman.
Nurs Stand. 1990 Aug 29-Sep 4;4(49):18. PMID: 2119747
- Wilson CW.
Myalgic encephalomyelitis: an alternative theory.
J R Soc Med. 1990 Aug;
83(8):481-3. PMID: 2231572
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1292769/pdf/jrsocmed00133-0005b.pdf
- Dowsett EG, Ramsay AM, McCartney RA, Bell EJ.
Myalgic encephalomyelitis--a
persistent enteroviral infection?
Postgrad Med J. 1990 Jul;66(777):526-30.
PMID: 2170962
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2429637/pdf/postmedj00163-0031.pdf
- Lynch S, Seth R.
Depression and myalgic encephalomyelitis.
J R Soc Med. 1990 May;
83(5):341. PMID: 2380955
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1292666/pdf/jrsocmed00136-0073a.pdf
- Shepherd C.
How I treat myalgic encephalomyelitis: forum.
Practitioner. 1990 Apr 8;234
(1486):326. PMID: 2371218
- Powell S.
Myalgic encephalomyelitis.
Br J Gen Pract. 1990 Apr;40(333):170. No
abstract available. PMID: 2115368
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1371257/pdf/brjgenprac00081-0042a.pdf
- Hodson AD.
Myalgic encephalomyelitis.
J R Soc Med. 1990 Mar;83(3):199-200. PMID:
20894766
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1292588/pdf/jrsocmed00138-0079c.pdf
- Myalgic encephalomyelitis.
J R Soc Med. 1990 Mar;83(3):199-200. No abstract
available. PMID: 2325071
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1292587/pdf/jrsocmed00138-0079b.pdf
- Prasher D, Smith A, Findley L.
Sensory and cognitive event-related potentials in myalgic
encephalomyelitis.
J Neurol Neurosurg Psychiatry. 1990 Mar;53(3):247-53. PMID:
2324756
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1014138/pdf/jnnpsyc00513-0063.pdf
- Grist NR.
Myalgic encephalomyelitis: postviral fatigue and the heart.
BMJ. 1989 Nov
11;299(6709):1219. PMID: 2513065
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1838100/pdf/bmj00258-0049b.pdf
- Lev M.
Myalgic encephalomyelitis.
J R Soc Med. 1989 Nov;82(11):693-4. PMID:
2593126
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1292388/pdf/jrsocmed00144-0069.pdf
- Snow P.
Myalgic encephalomyelitis.
N Z Med J. 1989 Aug 23;102(874):449.
- Murdoch JC.
The myalgic encephalomyelitis syndrome.
N Z Med J. 1989 Jul 26;102
(872):372-3. PMID: 2797553
- Coakley JH.
Myalgic encephalomyelitis and muscle fatigue.
BMJ. 1989 Jun 24;298
(6689):1711-2. PMID: 2503189
- Zala J.
Diagnosing myalgic encephalomyelitis.
Practitioner. 1989 Jun 22;233(1471):
916-9. PMID: 2594656
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1836756/pdf/bmj00237-0063d.pdf
- Myalgic encephalomyelitis.
BMJ. 1989 Jun 10;298(6687):1577-8. PMID: 2503125
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1836797/pdf/bmj00235-0051b.pdf
- Welch JC.
Abnormal erythrocytes in myalgic encephalomyelitis.
N Z Med J. 1989 Apr
26;102(866):202. PMID: 2710458
- Simpson LO.
Nondiscocytic erythrocytes in myalgic encephalomyelitis.
N Z Med J. 1989
Mar 22;102(864):126-7. PMID: 2927808
- Cant BR.
Myalgic encephalomyelitis.
N Z Med J. 1989 Feb 8;102(861):52. PMID:
2739970
- Willoughby E.
Myalgic encephalomyelitis.
N Z Med J. 1989 Jan 25;102(860):19-20.
PMID: 2913521
- Shepherd C.
Myalgic encephalomyelitis--is it a real disease?
Practitioner. 1989 Jan;233
(1461):41-2, 44, 46. PMID: 2798285
- Murdoch JC.
The myalgic encephalomyelitis syndrome.
Fam Pract. 1988 Dec;5(4):
302-6. Review. PMID: 2852613
- Myalgic encephalomyelitis.
N Z Med J. 1988 Nov 23;101(858):800-1. PMID:
3194079
- Hyde B, Bergmann S.
Akureyri disease (myalgic encephalomyelitis), forty years later.
Lancet. 1988 Nov 19;2(8621):1191-2. PMID: 2903396
- Willoughby EW.
Myalgic encephalomyelitis.
N Z Med J. 1988 Nov 9;101(857):773.
PMID: 3186037
- Spracklen FH.
The chronic fatigue syndrome (myalgic encephalomyelitis)--myth or
mystery?
S Afr Med J. 1988 Nov 5;74(9):448-52. Review. PMID: 3055363
- Myalgic encephalomyelitis.
N Z Med J. 1988 Oct 26;101(856 Pt 1):672. No abstract
available. PMID: 3186016
- Gordon N.
Myalgic encephalomyelitis.
Dev Med Child Neurol. 1988 Oct;30(5):677-82.
Review. PMID: 3068084
- Murdoch JC.
Cell-mediated immunity in patients with myalgic encephalomyelitis
syndrome.
N Z Med J. 1988 Aug 10;101(851):511-2. PMID: 3261407
- Myalgic encephalomyelitis, or what?
Lancet. 1988 Jul 9;2(8602):100-1. PMID:
2898668
- Holborow PL.
Pathophysiology of myalgic encephalomyelitis.
Med J Aust. 1988 Jun
6;148(11):598, 600. PMID: 3374430
- Lloyd AR, Wakefield D, Boughton C, Dwyer J.
What is myalgic encephalomyelitis?
Lancet. 1988 Jun 4;1(8597):1286-7. PMID: 2897549
- Archard LC, Bowles NE, Behan PO, Bell EJ, Doyle D.
Postviral fatigue syndrome:
persistence of enterovirus RNA in muscle and elevated creatine kinase.
J R Soc Med.
1988 Jun;81(6):326-9. PMID: 3404526
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1291623/pdf/jrsocmed00161-0020.pdf
- Bell EJ, McCartney RA, Riding MH.
Coxsackie B viruses and myalgic
encephalomyelitis.
J R Soc Med. 1988 Jun;81(6):329-31. PMID: 2841461
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1291624/pdf/jrsocmed00161-0023.pdf
- Jennekens FG, van Gijn J.
[Postviral fatigue syndrome or myalgic encephalomyelitis].
Ned Tijdschr Geneeskd. 1988 May 28;132(22):999-1001. Dutch. PMID: 3380192
- Wakefield D, Lloyd A, Dwyer J, Salahuddin SZ, Ablashi DV.
Human herpesvirus 6 and
myalgic encephalomyelitis.
Lancet. 1988 May 7;1(8593):1059. PMID: 2896906
- Maros K.
Myalgic encephalomyelitis?
Med J Aust. 1988 Apr 18;148(8):424. PMID:
3357477
- Lloyd A, Hanna DA, Wakefield D.
Interferon and myalgic encephalomyelitis.
Lancet. 1988 Feb 27;1(8583):471. PMID: 2893889
- Byrne E.
Idiopathic chronic fatigue and myalgia syndrome (myalgic encephalomyelitis):
some thoughts on nomenclature and aetiology.
Med J Aust. 1988 Jan 18;148(2):80-2.
PMID: 3336341
- Ho-Yen DO, Carrington D, Armstrong AA.
Myalgic encephalomyelitis and alpha-
interferon.
Lancet. 1988 Jan 16;1(8577):125. PMID: 2891971
- Behan PO, Behan WM.
Postviral fatigue syndrome.
Crit Rev Neurobiol. 1988;4(2):
157-78. Review. PMID: 3063394
- Matthew C.
Myalgic encephalomyelitis and the doctor.
N Z Med J. 1987 Sep 9;100(831):
569. PMID: 3451147
- Mukherjee TM, Smith K, Maros K.
Abnormal red-blood-cell morphology in myalgic
encephalomyelitis.
Lancet. 1987 Aug 8;2(8554):328-9. PMID: 2886780
- Archer MI.
The post-viral syndrome: a review.
J R Coll Gen Pract. 1987 May;37(298):
212-4. Review. PMID: 3320358
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1710789/pdf/jroyalcgprac00029-0021.pdf
- Matthew C.
Myalgic encephalomyelitis.
N Z Med J. 1986 Sep 10;99(809):678. PMID:
3463904
- Blackmore RJ.
Myalgic encephalomyelitis and Immunovir.
N Z Med J. 1986 Jul 9;99
(805):513. PMID: 3461389
- McCartney RA, Banatvala JE, Bell EJ.
Routine use of mu-antibody-capture ELISA for
the serological diagnosis of Coxsackie B virus infections.
J Med Virol. 1986 Jul;19(3):
205-12. PMID: 3016163
- Simpson LO, Shand BI, Olds RJ.
Blood rheology and myalgic encephalomyelitis: a pilot
study.
Pathology. 1986 Apr;18(2):190-2. PMID: 3093959
- Rowlandson PH, Stephens JA.
Cutaneous reflex responses recorded in children with
various neurological disorders.
Dev Med Child Neurol. 1985 Aug;27(4):434-47.
PMID: 2993087
- Staines D.
Myalgic encephalomyelitis hypothesis.
Med J Aust. 1985 Jul 22;143(2):91.
PMID: 4021881
- Byrne E, Trounce I, Dennett X.
Chronic relapsing myalgia (Post viral): clinical,
histological, and biochemical studies.
Aust N Z J Med. 1985 Jun;15(3):305-8. PMID:
3864422
- Maurizi CP.
Raphe nucleus encephalopathy (myalgic encephalomyelitis, epidemic
neuromyasthenia).
Med Hypotheses. 1985 Apr;16(4):351-4. PMID: 4010573
- Myalgic encephalomyelitis.
N Z Med J. 1985 Jan 23;98(771):20-1. PMID: 3855509
- Gow PJ.
Myalgic encephalomyelitis.
N Z Med J. 1984 Dec 12;97(769):868. PMID:
6595571
- Myalgic encephalomyelitis.
N Z Med J. 1984 Nov 14;97(767):782. PMID: 6593632
- Myalgic encephalomyelitis.
N Z Med J. 1984 Oct 10;97(765):698-9. PMID:
6592485
- Bell EJ, McCartney RA.
A study of Coxsackie B virus infections, 1972-1983.
J Hyg
(Lond). 1984 Oct;93(2):197-203. PMID: 6094660
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2129449/pdf/jhyg00014-0036.pdf
- Gow PJ.
Myalgic encephalomyelitis.
N Z Med J. 1984 Sep 12;97(763):620. PMID:
6591048
- Calder BD, Warnock PJ.
Coxsackie B infection in a Scottish general practice.
J R Coll
Gen Pract. 1984 Jan;34(258):15-9. PMID: 6319691
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1959663/pdf/jroyalcgprac00169-0017.pdf
- Keighley BD, Bell EJ.
Sporadic myalgic encephalomyelitis in a rural practice.
J R Coll
Gen Pract. 1983 Jun;33(251):339-41. PMID: 6310105
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1972871/pdf/jroyalcgprac00078-0021.pdf
- Fegan KG, Behan PO, Bell EJ.
Myalgic encephalomyelitis--report of an epidemic.
J R
Coll Gen Pract. 1983 Jun;33(251):335-7. PMID: 6310104
- Know your organizations: the Myalgic Encephalomyelitis Association.
Health Visit.
1982 Jul;55(7):350. PMID: 6921182
- Parish G.
Myalgic encephalomyelitis: faulty fibres?
Nurs Mirror. 1981 Oct 7;153(15):
41-2. PMID: 6913031
- Ramsay M.
Myalgic encephalomyelitis: a baffling syndrome.
Nurs Mirror. 1981 Oct
7;153(15):40-1. PMID: 6913030
- Parish JG.
Myalgic encephalomyelitis.
Lancet. 1981 Apr 25;1(8226):950-1. PMID:
6112360
- Layzer RB.
Myoglobinaemia in benign myalgic encephalomyelitis.
Lancet. 1981 Mar
21;1(8221):670. PMID: 6110899
- Goodwin CS.
Was it benign myalgic encephalomyelitis?
Lancet. 1981 Jan 3;1(8210):
37-8. PMID: 6109065
- May PG, Donnan SP, Ashton JR, Ogilvie MM, Rolles CJ.
Personality and medical
perception in benign myalgic encephalomyelitis.
Lancet. 1980 Nov 22;2(8204):1122-4.
PMID: 6107734
- Church AJ.
Myalgic encephalomyelitis.
Med J Aust. 1980 Aug 23;2(4):224. PMID:
7432298
- Behan PO.
Epidemic myalgic encephalomyelitis.
Practitioner. 1980 Aug;224(1346):
805-7. PMID: 7433399
- Bishop J.
Epidemic myalgic encephalomyelitis.
Med J Aust. 1980 Jun 14;1(12):585-6,
609. PMID: 7402153
- Ramsay AM, Rundle A.
Clinical and biochemical findings in ten patients with benign
myalgic encephalomyelitis.
Postgrad Med J. 1979 Dec;55(654):856-7. PMID: 548947 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2425703/?tool=pubmed
- Pampiglione G, Harris R, Kennedy J.
Electro-encephalographic investigations in
myalgic encephalomyelitis.
Postgrad Med J. 1978 Nov;54(637):752-4. PMID: 746023
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1606252/pdf/brmedj00135-0058a.pdf
- Behan PO.
Post-infectious encephalomyelitis: some aetiological mechanisms.
Postgrad
Med J. 1978 Nov;54(637):755-9. PMID: 34143
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1606252/pdf/brmedj00135-0058a.pdf
- Wookey C.
Epidemic myalgic encephalomyelitis.
Br Med J. 1978 Jul 15;2(6131):202.
PMID: 678851
- Epidemic myalgic encephalomyelitis.
Br Med J. 1978 Jun 3;1(6125):1436-7.
PMID: 647324
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1604957/pdf/brmedj00128-0006b.pdf
- Ramsay AM, Dowsett EG, Dadswell JV, Lyle WH, Parish JG.
Icelandic disease (benign
myalgic encephalomyelitis or Royal Free disease)
Br Med J. 1977 May 21;1(6072):
1350. PMID: 861618
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1607215/pdf/brmedj00463-0058b.pdf
- Parish JG.
Benign myalgic encephalomyelitis.
Br J Psychiatry. 1973 Jun;122(571):735.
PMID: 4716076
- Ramsay AM.
Benign myalgic encephalomyelitis.
Br J Psychiatry. 1973 May;122(570):
618-9. PMID: 4717041
- Walther H.
[Epidemic myalgic encephalomyelitis].
Schweiz Rundsch Med Prax. 1972
Apr 11;61(15):469-80. German. PMID: 5022278
- Benign myalgic encephalomyelitis.
Med J Aust. 1970 Jul 4;2(1):3. PMID: 5447847
- Lyle WH.
Encephalomyelitis resembling benign myalgic encephalomyelitis.
Lancet.
1970 May 23;1(7656):1118-9. PMID: 4191997
- Innes SG.
Encephalomyelitis resembling benign myalgic encephalomyelitis.
Lancet.
1970 May 9;1(7654):969-71. PMID: 4191935
- Mourad S, Chidiac J.
Benign myalgic encephalomyelitis in Lebanon.
J Med Liban.
1969;22(6):735-40. PMID: 5370523
- Kendell RE.
The psychiatric sequelae of benign myalgic encephalomyelitis.
Br J
Psychiatry. 1967 Aug;113(501):833-40. PMID: 6048369
- Gsell O.
[Benign myalgic encephalomyelitis, epidemic pseudoneurasthenia].
Schweiz
Med Wochenschr. 1963 Feb 2;93:197-200. German. PMID: 13950994
- Price JL.
Myalgic encephalomyelitis.
Lancet. 1961 Apr 8;1(7180):737-8. PMID:
13737972
- Pool JH, Walton JN, Brewis EG, Uldall PR, Wright AE, Gardner PS.
Benign myalgic
encephalomyelitis in Newcastle upon Tyne.
Lancet. 1961 Apr 8;1(7180):733-7. PMID:
13737057
- Klajman A, Pinkhas B, Rannon L.
[An outbreak of an epidemic of benign myalgic
encephalomyelitis].
Harefuah. 1960 May 15;58:314-5. Hebrew. PMID: 14409571
- Bornstein B, Bechar M, Lass H.
Benign myalgic encephalomyelitis. (Report of five
cases).
Psychiatr Neurol (Basel). 1960 Mar;139:132-40. PMID: 13802904
- Daikos GK, Garzonis S, Paleologue A, Bousvaros GA, Papadoyannakis N.
Benign
myalgic encephalomyelitis: an outbreak in a nurses' school in Athens.
Lancet. 1959 Apr
4;1(7075):693-6. PMID: 13642848
- Acheson ED.
The clinical syndrome variously called benign myalgic encephalomyelitis,
Iceland disease and epidemic neuromyasthenia.
Am J Med. 1959 Apr;26(4):569-95.
PMID: 13637100
http://www.meresearch.org.uk/information/keypubs/Acheson_AmJMed.pdf
- Bhatia BB, Chandra S, Bhushan C.
Benign myalgic encephalomyelitis.
J Indiana State
Med Assoc. 1958 Oct;31(8):327-8. PMID: 13611265
- Gsell O.
[Encephalitis myalgica epidemica, a poliomyelitis-like disease; epidemic
neuromyasthenia, benign myalgic encephalomyelitis].
Schweiz Med Wochenschr. 1958
May 17;88(20):488-91. German. PMID: 13568694
- Greene IM.
Benign myalgic encephalomyelitis; syndrome mimicking anterior
poliomyelitis.
J Fla Med Assoc. 1958 Apr;44(10):1105-6. PMID: 13525606
- Galpin JF.
Benign myalgic encephalomyelitis.
Br J Clin Pract. 1958 Mar;12(3):186-8
passim. PMID: 13510534
- Deisher JB.
Benign myalgic encephalomyelitis (Iceland disease) in Alaska.
Northwest
Med. 1957 Dec;56(12):1451-6. PMID: 13484090
- [Benign epidemic myalgic encephalomyelitis].
Recenti Prog Med. 1957 Nov;23(5):
525-31. Italian. PMID: 13518620
- EPIDEMIC myalgic encephalomyelitis.
Br Med J. 1957 Oct 19;2(5050):927-8.
PMID: 13472011
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1962482/pdf/brmedj03125-0047.pdf
- Acheson ED.
Benign myalgic encephalomyelitis.
Lancet. 1957 Apr 20;272(6973):834-5.
PMID: 13417614
- Galpine JF, Brady C.
Benign myalgic encephalomyelitis.
Lancet. 1957 Apr 13;272
(6972):757-8. PMID: 13417586
- Lindan R.
Benign Myalgic Encephalomyelitis.
Can Med Assoc J. 1956 Oct 1;75(7):
596-7. PMID: 20325349
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1824640/pdf/canmedaj00742-0051.pdf
- Blattner RJ.
Benign myalgic encephalomyelitis (Akureyri disease, Iceland disease).
J
Pediatr. 1956 Oct;49(4):504-6. PMID: 13358047
- A new Clinical Entity ? Lancet 1956
- Outbreak at the Royal Free.
E.D Acheson. The Lancet, Volume 266, Issue 6886, Pages 394 - 395, 20 August 1955.
Years of Epidemics
1917 Van Economo reports an illness involving brain and neurological inflammation and great fatigue and some deaths. See paper 'New Clinical Entity' published in the Lancet in 1956.
1918 - 1924, several outbreaks of an illness involving brain and neurological inflammation and fatigue reported throughout Europe. See paper 'New Clinical Entity' published in the Lancet in 1956.
1924 England and Wales 5,039 cases of encephalitis lethargica. See paper 'New Clinical Entity' published in the Lancet in 1956.
1934
Los Angeles County Hospital. Called 'Atypical Poliomyelitis'
1936
Fond Du Lac, Wisconsin - St. Agnes Convent - Encephalitis
1937
Erstfeld, Switzerland -
Abortive Poliomyelitis
1937
St. Gallen, Switzerland
- Frohburg Hospital – Abortive Poliomyelitis
1939
Middlesex, England - Harefield Sanatorium
1939
Degersheim, Switzerland - Abortive Poliomyelitis
1945
Pennsylvania. Hospital of the University of Pennsylvania - epidemic Pleurodynia
1946
Iceland
disease resembling Poliomyelitis with the character of Akureyri disease
1948
Iceland, North Coast towns - epidemic simulating Poliomyelitis
1949
Adelaide, South Australia - a disease resembling Poliomyelitis
1949 Cambridgeshire, England -
aberrant poliomyelitis. Involvement of other Enteroviruses suspected.
1950
Louisville, Kentucky -- St. Joseph
's Infirmary - epidemic Neuromyasthenia
1950
Upper State New York -- outbreak resembling the
Iceland disease, simulating
"
acute Anterior Poliomyelitis
1952
London, England - Middlesex Hospital Nurses
'
Home - Encephalomyelitis
associated with Poliomyelitis virus
1952
Copenhagen, Denmark - epidemic Myositis
1952
Lakeland, Florida - epidemic Neuromyasthenia
1953
Coventry and District, England - an illness resembling Poliomyelitis observed in
nurses
1953
Rockville, Maryland - Chestnut Lodge Hospital - Poliomyelitis-like epidemic
Neuromyasthenia
1953
Jutland, Denmark - epidemic Encephalitis with vertigo
1954 Seward, Alaska - benign Myalgic Encephalomyelitis (Iceland Disease)
1954
Berlin, Germany - British army - further outbreak of a disease resembling
Poliomyelitis
1954
Liverpool, England - outbreak among medical and nursing staff in a local
hospital
1955
Dalston, Cumbria, England – epidemic and sporadic outbreak of an unusual
disease
1955
London, England - Royal Free Hospital - outbreak in staff and patients of Benign
Myalgic Encephalomyelitis
1955 Hampstead, London
1955
Perth, Australia - virus epidemic in waves
1955
Gilfac Goch, Wales - outbreak of benign Myalgic Encephalomyelitis
1955
Durban City, South Africa - Addington Hospital - outbreak among nurses of Durban Mystery Disease
1955
Segbwema, Sierra Leone - outbreak of Encephalomyelitis
1955
Patreksfjorour and Porshofn, Iceland - unusual response to polio vaccine
1955
Northwest London, England - nurses
'
residential home - acute Infective
Encephalomyelitis simulating poliomyelitis
1956
Ridgefield, Connecticut - epidemic Neuromyasthenia
1956
Punta Gorda Florida - outbreak of epidemic Neuromyasthenia
1956
Newton-le-Willows, Lancashire, England - Lymphocytic Meningoencephalitis with
myalgia and rash
1956
Pittsfield and Williamstown, Massachusetts - benign Myalgic Encephalomyelitis
1956
Coventry, England - epidemic malaise, benign Myalgic Encephalomyelitis
1957
Brighton, South Australia - Cocksakie Echo virus Meningitis, epidemic Myalgic
Encephalomyelitis
1958
Athens, Greece - nurses
'
school - outbreak of benign Myalgic Encephalomyelitis
with periostitis and arthopathy noted.
1958
Southwest London, England - reports of sporadic cases of Myalgic
Encephalomyelitis
1959
Newcastle Upon Tyne, England - outbreak of benign Myalgic Encephalomyelitis
1961
Basel, Switzerland - sporadic cases of benign Myalgic Encephalomyelitis
1961
New York State - outbreak of epidemic Neuromyasthenia in a convent
1964
Northwest London, England - epidemic malaise, epidemic Neuromyasthenia
1964
Franklin, Kentucky - outbreak of Neuromyasthenia in a factory
1967
Edinburgh, Scotland - sporadic cases resembling benign Myalgic
Encephalomyelitis
1968
"
Fraidek, Lebanon - benign Myalgic Encephalomyelitis
1969
Brooklyn, New York - State University of New York Downstate Medical Center -
epidemic Neuromyasthenia, unidentified symptom complex
1970
Lackland Air Force Base, Texas - epidemic Neuromyasthenia
1970
London, England - Great Ormond Street Hospital for Children - outbreak of
Neuromyasthenia among nurses
1975
Sacramento, California - Mercy San Juan Hospital - Infectious Venulitis, epidemic
"
Phelobodynia
1976
Southwest Ireland - epidemic Neuromyasthenia, benign Myalgic
Encephalomyelitis
1977
Dallas – Fort Worth, Texas - epidemic Neuromyasthenia
1979
Southampton, England - Myalgic Encephalomyelitis
1980
West Kilbridge, Ayrshire, Scotland - epidemic Myalgic Encephalomyelitis
1980
San Francisco, California – epidemic persistent flu-like illness
1981
Stirlingshire, Scotland
- sporadic Myalgic Encephalomyelitis
1982
West Otago, Dunedin and Hamilton, New Zealand - Myalgic Encephalomyelitis
1983
Los Angeles, California - initial cases of an unknown, chronic symptom complex
involving profound "fatigue"
1984
Lake Tahoe Area of California/Nevada - start of a yearlong epidemic involving
"
over 160 cases of chronic illness eventually characterized as Chronic Fatigue
Syndrome
Source: Paradigm Change web site
|





